Enzymes and the active site
As a kid, I wore glasses and desperately wanted a pair of contact lenses. When I was finally allowed to get contacts, part of the deal was that I had to take very, very good care of them, which meant washing them with cleaner every day, storing them in a sterile solution, and, once a week, adding a few drops of something called “enzymatic cleaner.” I didn’t know exactly what “enzymatic cleaner” meant, but I did learn that if you forgot you’d added it and accidentally put your contacts in your eyes without washing them, you were going to have burning eyes for a good fifteen minutes.
As I would later learn, all that “enzymatic” meant was that the cleaner contained one or more enzymes, proteins that catalyzed particular chemical reactions – in this case, reactions that broke down the film of eye goo that accumulated on my contacts after a week of use. (Presumably, the reason it stung when I got it in my eyes was that the enzymes would also happily break down eye goo in an intact eye.) In this article, we’ll look in greater depth at what an enzyme is and how it catalyzes a particular chemical reaction.
Enzymes and activation energy
A substance that speeds up a chemical reaction—without being a reactant—is called a catalyst. The catalysts for biochemical reactions that happen in living organisms are called enzymes. Enzymes are usually proteins, though some ribonucleic acid (RNA) molecules act as enzymes too.
Enzymes perform the critical task of lowering a reaction's activation energy—that is, the amount of energy that must be put in for the reaction to begin. Enzymes work by binding to reactant molecules and holding them in such a way that the chemical bond-breaking and bond-forming processes take place more readily.
To clarify one important point, enzymes don’t change a reaction’s ∆G value. That is, they don’t change whether a reaction is energy-releasing or energy-absorbing overall. That's because enzymes don’t affect the free energy of the reactants or products.
Instead, enzymes lower the energy of the transition state, an unstable state that products must pass through in order to become reactants. The transition state is at the top of the energy "hill" in the diagram above.
Active sites and substrate specificity
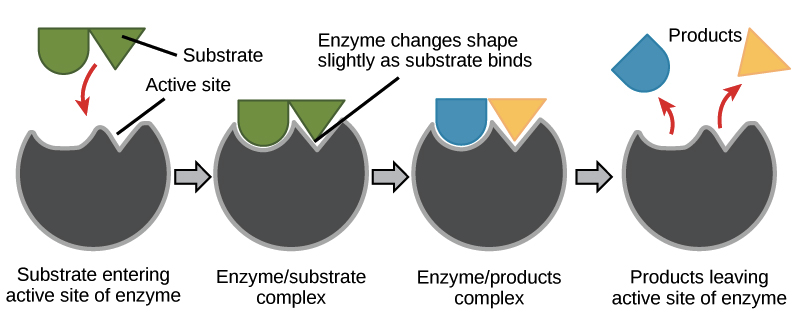
To catalyze a reaction, an enzyme will grab on (bind) to one or more reactant molecules. These molecules are the enzyme's substrates.
In some reactions, one substrate is broken down into multiple products. In others, two substrates come together to create one larger molecule or to swap pieces. In fact, whatever type of biological reaction you can think of, there is probably an enzyme to speed it up!
The part of the enzyme where the substrate binds is called the active site (since that’s where the catalytic “action” happens).
